On Tuesday 13 April, Health Minister Dr Zwelini Mkhize announced the suspension of the Johnson & Johnson (J&J) vaccine roll-out to healthcare workers over fears of blood clotting. The decision comes after the Food and Drug Administration (FDA) in the United States suspended its use of the vaccine and J&J’s delay in further shipments. Over 1.5 million South Africans have been infected with COVID-19 and the variant B1.351, causing the loss of over 53 000 lives. After an incredibly difficult year for South Africans, the arrival of the COVID-19 vaccine roll-out brings new hope for life returning to some degree of normality. The pandemic has highlighted the socio-economic and healthcare inequalities found across South African communities. Effectively administering the COVID-19 vaccines will be vital in the quest to halt further socio-economic decline and give South Africans a brighter outlook for 2021.
Johnson & Johnson vaccine vials against the COVID-19 coronavirus are seen at the Klerksdorp Hospital as South Africa proceeds with its inoculation campaign on February 18, 2021. (Photo by Phill Magakoe / AFP)
Despite facing serious challenges in Personal Protective Equipment (PPE) and oxygen availability, and overwhelming hospital admissions, South African healthcare workers must be commended for their efforts in combatting the virus thus far. Their efforts have translated to a recovery rate of about 95% among COVID-19 patients. It is hoped that the vaccine roll-out will begin easing pressure on our healthcare system, because there are many other important healthcare crises that need our healthcare workers’ attention.
Progress for Phase 1
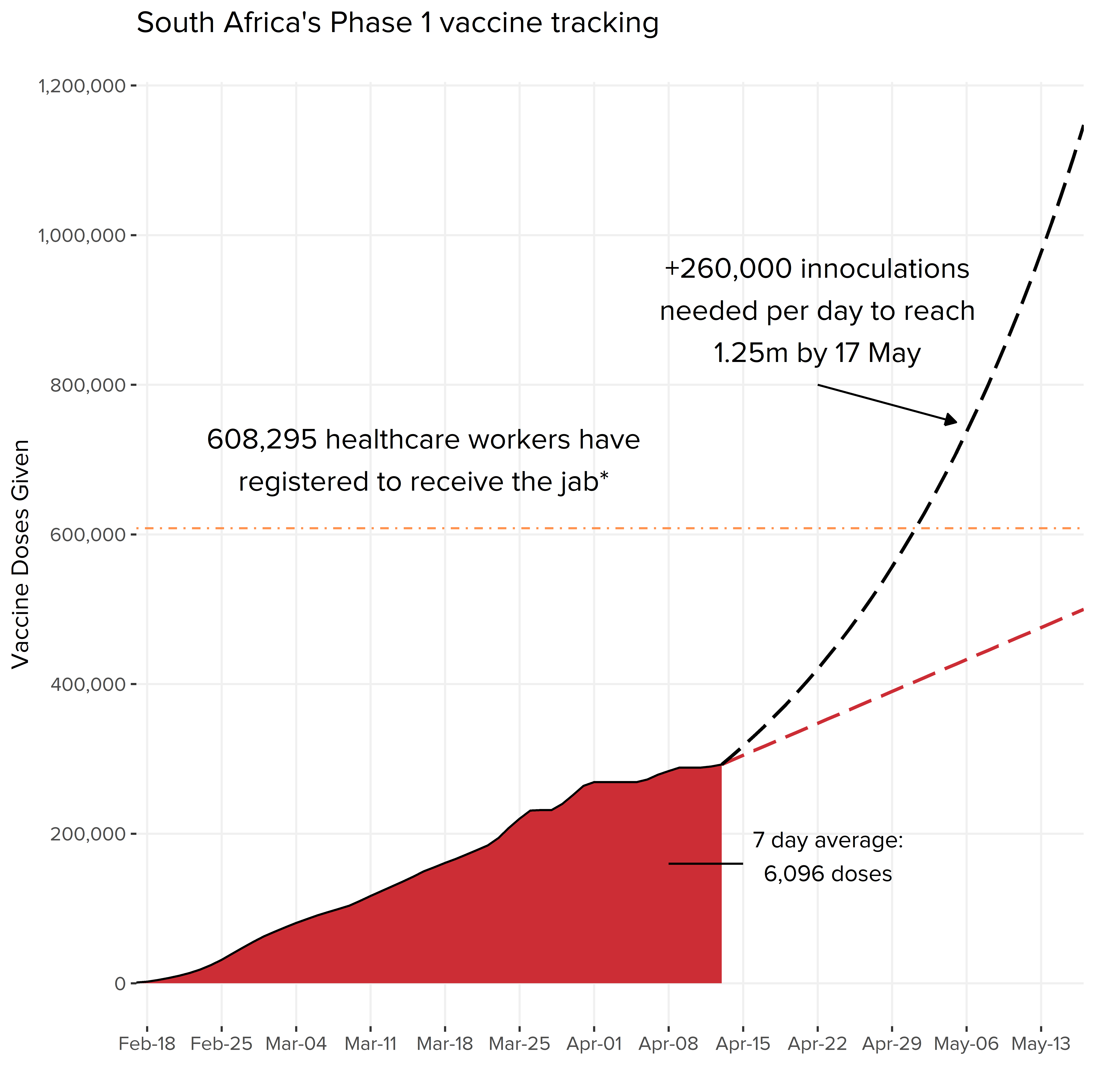
Source: Department of Health, 15 April 2021. *Announced on 24 March 2021
South Africa was the first country on the African continent to receive the AstraZenca COVID-19 vaccine in February. Since then, Minister Mkhize presented a three-phase plan to inoculate healthcare workers, essential workers, vulnerable groups, and the public. The first phase of the vaccine roll-out runs in conjunction with the Sisonke Study, which is a clinical trial of the single-dose J&J COVID-19 vaccine. The clinical trial allows the government to make the J&J vaccine available to healthcare workers while it processes the licensing of the vaccine. The target set for the Sisonke trail is to vaccinate 500,000 healthcare workers.
Since vaccinations began in February, about 292,623 healthcare workers have received their dose. This slow rate of inoculation has raised concerns. The daily vaccination rate is too low to meet the proposed target of 1.25 million healthcare workers by 17 May. However, at the current rate we will only vaccinate just over 500,000 healthcare workers. When President Cyril Ramaphosa addressed the nation before the Easter weekend, he indicated that the government were on track to complete healthcare worker vaccinations within three months. However, there is confusion as to whether he is referring to the Sisonke trial or the 1.25 million targets. In Minister Mkhize’s cabinet statement, he refers to 1.5 million healthcare workers, whereas on the National Institute for Communicable Diseases (NICD) website, the number is 1.2 million healthcare workers. That would leave around 650,000 healthcare workers remaining, to be carried over to Phase 2, placing an additional burden on the roll-out process. Strengthening our primary healthcare infrastructure is vital if we are to vaccinate 67% (~41 million people) of South Africa’s population.

Source: Department of Health, Government Communications 2021
Reflections from healthcare workers
In an attempt to ascertain the vaccine roll-out’s progress, we interviewed some healthcare workers – doctors, administrative staff and biokineticists. Experiences varied and were mostly positive. The electronic vaccination data system (EVDS) was complimented for its ease of use in both registering and booking their vaccine appointments. In some cases, doctors were turned away if doses had run out before the end of the day, requiring them to reschedule for another day. When queuing for their vaccination, social distancing protocols were not strictly followed, which were a concern for those we spoke to. They all described having the expected post-vaccination symptoms for 48-72 hours.
A repeated concern raised was the hesitancy among some healthcare workers to register for vaccination. There are myths and misinformation circulating amongst communities about the safety of the vaccine. If healthcare workers are hesitant, rallying citizens to register could be a challenge. The government has released a ‘myths and facts’ page addressing some of this misinformation. The government implemented mask-wearing campaign is a good example of a positive initiative adopted by the public and private sector. Continued communication from government and civil society to remote communities is equally important to build positive vaccine sentiment.
Minister Mkhize has also proposed a post market surveillance system study to ensure medical authorities closely monitor the deployment of both J&J and Pfizer.
Three phase roll-out strategy – the devil is in the detail
Phase 1 roll-out facts and challenges
The first phase of the government’s roll-out plan proposed 18 centralised sites which have now expanded to 58. During the expansion there was confusion around certain sites being listed as open but were in fact closed and vice versa. For a venue to be utilised as a vaccination site, the South African Medical Research Council (SAMRC), the Department of Health (DoH), Desmond Tutu Health Foundation, Centre for Aids Programme of Research (CAPRISA) and Janssen Pharmaceuticals must be consulted. This makes the coordination of opening a vaccination site somewhat complex. The process of approving venues creates a bottleneck. The sites need constant updating on the EVDS where registration occurs. There is little information yet on how the government plans to overcome this process when Phase 2 begins next month, which could add to the already strained roll-out process.
Phase 2 roll-out plans
The government plans on increasing the number of vaccination sites to 1,750 and they will range between small, medium and large sites. Small sites will likely be community clinics or pharmacies and general practitioner offices. Medium sites include hospitals, medical centres and retail locations that may be fixed or temporary. Large sites are venues such as stadiums and conference centres.
A massive scale-up effort is needed for site approval if they are to reach this target and vaccinate up to 300,000 people a day. To reach that number per day we will need to mobilise at least 6,250 vaccinators (per day) and they’ll need to do 48 inoculations a day. If South Africa enters a third wave it could mean that some sites would have to de-escalate vaccination efforts to open facilities for COVID-19 testing, slowing down the roll-out further.
Vaccine procurement
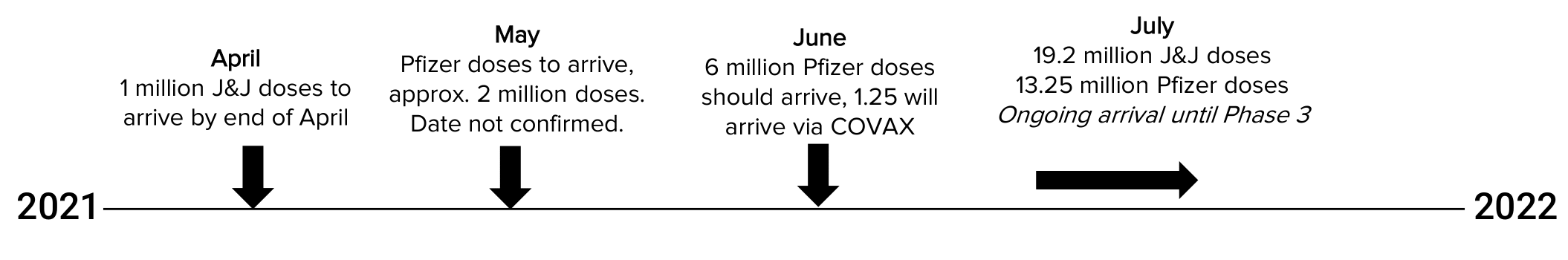
Source: Department of Health 2021
Before the end of April, an additional one million doses of J&J are expected to arrive, after which two tranches of 900,000 doses will arrive in May and June. 31 million J&J (single dose) and 30 million Pfizer (two dose) vaccines have been secured by the DoH. Just under two million Pfizer doses are expected to arrive in May. In total South Africa is expected to have 46 million full vaccine doses, which should be sufficient to cover the necessary 40 million needed to obtain herd immunity.
The J&J vaccine will be particularly important for the roll-out in rural areas and remote communities. The J&J vaccine is easier to store, with only a single dose and can be frozen and kept for up two years (refer to the table below). Its efficacy against the B1.351 variant is 95% and is very effective in preventing severe disease or death associated with COVID-19. Unopened vials can be kept for up to three months at fridge temperatures of between 2˚C and 8˚C. If they are drawn into syringes, they will need to be administered within six hours.
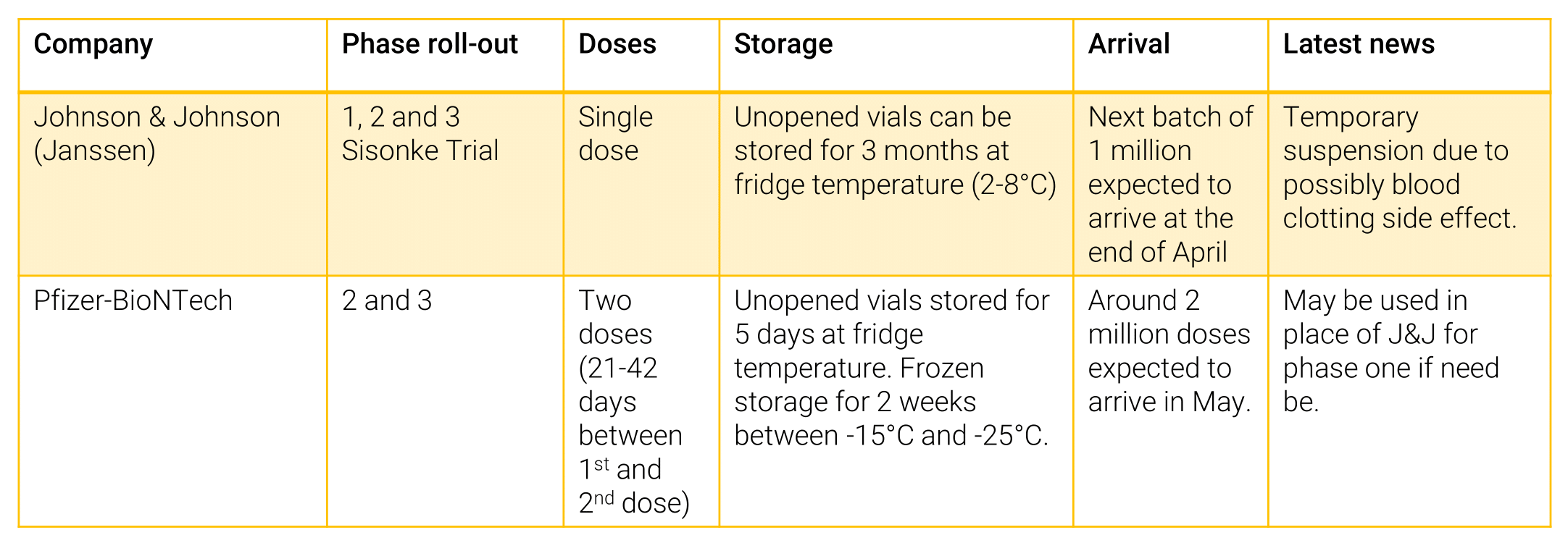
Source: Department of Health, National Institute of Communicable Diseases 2021
The Pfizer vaccine on the other hand requires colder storage -20˚C for up to 14 days, and 2-8˚C up to 5 days, with two doses required and can be frozen for up to 6 months (refer to table below). The second dose should be administered between 21-42 days after the first dose. As yet there is no substantial clinical evidence that the Pfizer vaccine protects against the B1.351 variant. A recent study concluded that the B1.351 variant was, to some degree, able to break through’s the Pfizer vaccine’s protection. The government will need to confirm veracity of this study before administering the vaccine. Coordination amongst pharmacists, vaccinators, researchers, and medical administration staff is vital to ensure jabs are not wasted, so as to avoid any impediments to the roll-out process.
Supply chain governance
Proposals for storage and distribution of the vaccine will be done on an open-tender basis. The Biologicals and Vaccines Institute of Southern Africa (Biovac) and the SAMRC have helped distribute the J&J vaccine but the government is yet to announce who will distribute the Pfizer vaccine. Minister Mkhize has said the department have identified storage capacity across the country but no further details on where these facilities are located.
When vaccines reach their destination, they will need to be signed off by a pharmacist on delivery. The World Health Organization (WHO) recommends at least one pharmacist for every 2,300 people, in South Africa we have one for every 3,837 people. The distribution of pharmacists across provinces is not equal and may pose a problem when delivery is made to areas without these specialists. Measures should be implemented to ensure the adequate placement of pharmacists across provinces is finalised so as to mitigate challenges that may arise.
Local vaccine manufacturing
The government has partnered with Biovac, Aspen and ImmunityBio to develop localised manufacturing of a COVID-19 vaccine that will offer long duration immunity against multiple variants of COVID-19. President Ramaphosa has called for the continent to use its existing skills and capacity to manufacture its own vaccines. Rwanda’s President Paul Kagame said that “vaccine equity” cannot be guaranteed by “goodwill alone” and that Africa should produce its own vaccines and pharmaceuticals products. The COVID-19 pandemic presents an opportunity for African countries to collaborate and promote continental (and local) innovation within the pharmaceutical industry.
Recommendations
- Government to continue sharing relevant information learnt from Phase 1 to grow public trust and confidence in the vaccination roll-out process.
- Finalise the sites and required number of healthcare workers to be placed to administer vaccines per site.
- Formulate measures to overcome potential internet connectivity concerns for vaccine registration.
- Finalise registration process for undocumented migrants without passports or identification documents.
- Government to continue sharing the vaccination roll-out progress.
[1] Current population estimate for South Africa is 59.6 million according to StatsSA data from 2020.












What is the honest truth about the Covid vacinations. Who can I hold accountable if I die within 5 years due to taking the vacination.